Unlocking the promise of mRNA therapeutics
Nature Biotechnology (2022)
Abstract
The extraordinary success of mRNA vaccines against coronavirus disease 2019 (COVID-19) has renewed interest in mRNA as a means of delivering therapeutic proteins. Early clinical trials of mRNA therapeutics include studies of paracrine vascular endothelial growth factor (VEGF) mRNA for heart failure and of CRISPR–Cas9 mRNA for a congenital liver-specific storage disease. However, a series of challenges remains to be addressed before mRNA can be established as a general therapeutic modality with broad relevance to both rare and common diseases. An array of new technologies is being developed to surmount these challenges, including approaches to optimize mRNA cargos, lipid carriers with inherent tissue tropism and in vivo percutaneous delivery systems. The judicious integration of these advances may unlock the promise of biologically targeted mRNA therapeutics, beyond vaccines and other immunostimulatory agents, for the treatment of diverse clinical indications.
Conclusion
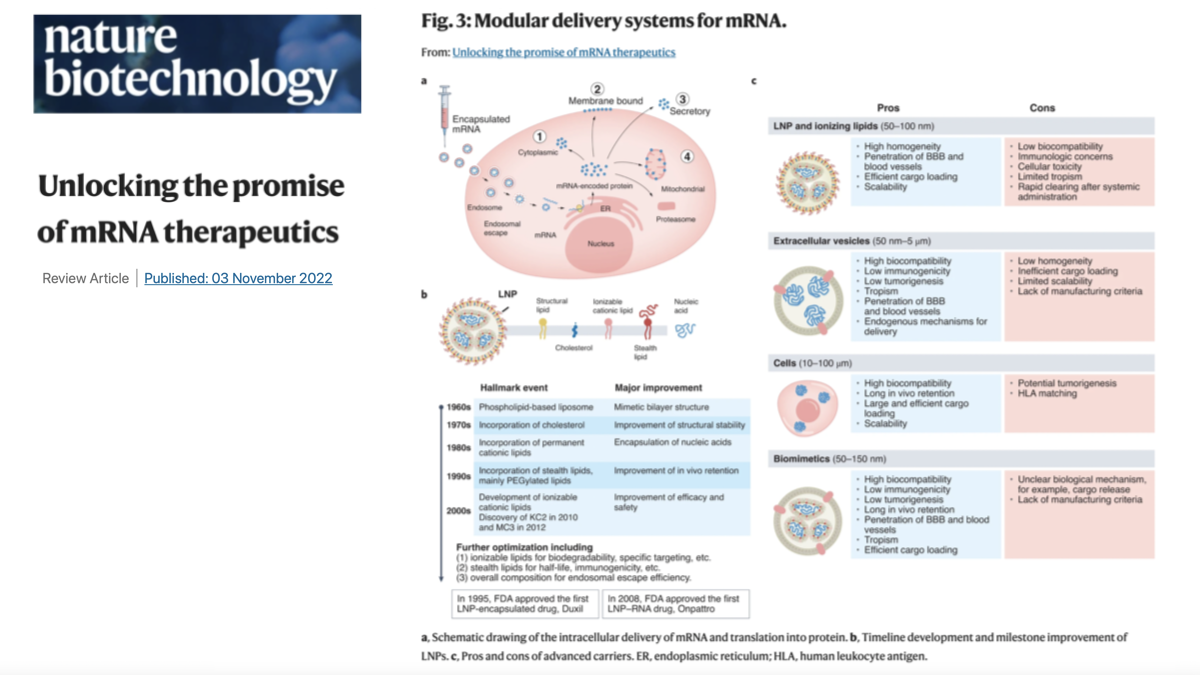
Three decades of scientific and clinical advances coupled with a massive effort to develop mRNA COVID-19 vaccines bodes well for the future of mRNA therapeutics. As noted above, mRNA encoding any protein can be quickly made at clinical grade with a few clicks in an automated, scalable, cell-free format. In the near future, it should be possible to generate modular, scalable Good Manufacturing Practice (GMP)-level manufacturing units that can be set up in any GMP-level facility, removing the need for cold chain transport. In this regard, lyophilization of mRNA therapeutics is on the horizon and will largely obviate distribution issues that exist with current COVID-19 mRNA vaccines. As new LNP and non-LNP carriers are developed with improved side effect profiles and increased capacity, complex gene and base editing may become feasible, along with repeated administration, to foster a new approach to enzyme replacement therapy.
At the same time, it is instructive to recall a cautionary tale from the history of recombinant protein therapy. In the early days of this field, it was projected that most growth factors would become drugs. Thirty years after the cloning of VEGF, it remains to be seen if it will become a clinically valuable therapeutic. Accordingly, the future of mRNA drugs may depend on matching this ‘software of life’ to the biological ‘hardware’ of human physiological systems with increased precision, longer duration and options for chronic dosing with tolerable safety profiles. In the coming years, rapid developments in the mRNA cargo, intracellular carriers and in vivo delivery systems, coupled with deep biological and clinical insight and intuition, should offer new hope for the many patients with unmet clinical needs that cannot easily be addressed by other therapeutic modalities.